Abstract
Introduction:
Approximately one-third of patients (pts.) with diffuse large B-cell lymphoma (DLBCL) develop relapsed or refractory (R/R) disease after frontline rituximab-(R) based chemo immunotherapy. Variability in expression of the B-cell surface antigen CD20, and possibly CD19, is thought to be an important mechanism of treatment failure, but there is vast heterogeneity in the reported incidence, with most reports utilizing Immunohistochemical (IHC) staining which is non-quantitative in nature. New therapeutics including CAR T-cells, antibodies (Ab) (tafasitimab) and Ab-drug conjugates (loncastuximab teserine) target CD19, while several bispecific engager Abs. in development target CD20. Understanding the evolution of target antigen expression in R/R DLBCL can provide information regarding selection and sequencing of therapies. We sought to quantitate the change in surface expression of CD19 and CD20 in R/R DLBCL after R-based chemo immunotherapy relative to pretreatment levels, to provide insight into the relative stability of these targets.
Methods:
Pts. diagnosed with DLBCL after January 2014, who were R/R to frontline R-based chemo immunotherapy (R-CHOP or R-EPOCH) were retrospectively identified. Pt. and disease related clinical variables at diagnosis (Dx) and first R/R were recorded. Data from IHC were obtained, as were archival flow cytometry assays performed on tumor biopsies at Dx and in the R/R setting. Using FCS Express® software, neoplastic cells were gated, and fluorescence intensity (FI) of CD19 and CD20 expression were reported (using fluorochromes APC-A for CD19 and PECy7 for CD20, respectively). Multivariate linear mixed model was used to compare median and geometric mean FI of CD19 and CD20 between Dx and R/R, while adjusting for other clinical variables.
Results:
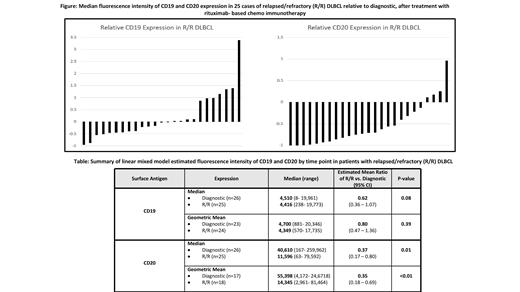
A total of 51 flow cytometry assays (26 Dx and 25 R/R) were analyzed for 33 pts. Median age at Dx was 64 (range, 41-76) yrs., 24 pts. (73%) were male and 29 (88%) had IPI ≥ 2. 11 (33%) pts. had a prior indolent lymphoma. Cell of origin at Dx was GCB in 16 (49%) and non-GCB in 12 (36%) pts., while two had a double-hit rearrangement at diagnosis. Treatment was R-CHOP in 27 cases (82%) and R-EPOCH in 6 (18%). Median time to R/R was 10.4 months.
There was a significant reduction in median FI of CD20 from Dx [median: 40,610 (range: 167 - 259,962)] to R/R [median: 11,596 (range: 63 - 79,592)], representing a mean reduction of 63% at R/R relative to Dx (P= 0.01; 95% CI: 20-73%). Similar change was observed in geometric mean FI of CD20, which was reduced 65% at R/R relative to Dx (P< 0.01; 95%CI: 31-82%). In comparison, on IHC, CD20 was reported to be negative at R/R relative to Dx in only one of 16 pts. for whom IHC results were available. Median and geometric mean FI of CD19 at R/R were 38% and 20% lower compared to Dx, respectively, but these differences were not statistically significant (P= 0.08 and 0.39, respectively) (Table). When examining the relative change in FI at R/R in individual cases, compared to the mean FI of all Dx cases, we observed that 21 out 25 R/R cases (84%) had reduction of CD20 whereas only 14/25 (56%) had reduction of CD19. Interestingly, 7/25 (28%) R/R cases had an increase in CD19 expression by >80% (Figure).When adjusting for clinical variables such as age, sex, presence of B symptoms, bone marrow (BM) involvement, PS and prior indolent lymphoma, the change in CD20 median FI from Dx to R/R maintained statistical significance (p=0.01). Reduction in CD20 geometric mean FI from Dx to R/R was significantly associated with age >60 years (p=0.04), BM involvement (p <0.01) and >1 site of extra nodal involvement (p= 0.03) at Dx.
Conclusions:
Quantitative assessment by flow cytometry revealed a significant decline in expression of CD20 at R/R compared to Dx in the majority of patients with DLBCL treated with R-based chemo immunotherapy. CD19 expression was unchanged in most R/R cases but was found to be dramatically upregulated in a subset of R/R cases. Given the role of CD19 mediated pathways in B-cell NHL and its association with PI3K pro-survival signaling, these data merit further exploration as a potential mechanism of treatment resistance. These findings also highlight the importance for repeat tissue biopsy at the time of suspected R/R DLBCL, with focus on therapeutic target expression, as it may influence treatment decisions or enrollment in clinical trials exploring efficacy of newer agents.
Caimi: TG Therapeutics: Honoraria; Seattle Genetics: Consultancy; Amgen Therapeutics.: Consultancy; XaTek: Patents & Royalties: Royalties from patents (wife); Verastem: Consultancy; Genentech: Research Funding; Kite Pharmaceuticals: Consultancy; ADC Theraputics: Consultancy, Research Funding. Hill: Celgene (BMS): Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel Support, Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding; AstraZenica: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Gentenech: Consultancy, Honoraria, Research Funding; Incyte/Morphysis: Consultancy, Honoraria, Research Funding; Beigene: Consultancy, Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal